Abstract
Introduction: Thrombocytopenia is a major dose-limiting hematologic toxicity of anti-myeloma therapy. Clinical trial data in the relapsed/refractory setting have linked the development of thrombocytopenia during therapy with bortezomib with improved response to treatment. Less is known about the prognostic significance of early treatment related thrombocytopenia during initial therapy for newly diagnosed multiple myeloma (MM) using novel agents.
Aims: The aim of this study was to characterize the effect of new, treatment related thrombocytopenia during initial therapy for newly diagnosed MM with novel agents on survival and clinical response.
Methods: We identified 690 patients with newly diagnosed MM treated at Mayo Clinic, Rochester from January 2004 to December 2015 through a retrospective record review. All patients received novel agents including proteasome inhibitors (PI) and/or immunomodulatory drugs, were evaluable using the International Myeloma Working Group uniform response criteria, and had platelet counts greater than 150,000/µL prior to initial treatment. Survival outcomes and response were compared in patients who became thrombocytopenic within two cycles of first line treatment, defined as a nadir platelet count less than 150,000/µL, and in patients who did not develop thrombocytopenia. Survival estimates were calculated using the Kaplan-Meier method, and the log-rank test was used to compare survival in subgroups. Multivariable-adjusted proportional hazards regression models were fit to adjust for known prognostic factors including age, sex, International Staging System (ISS) stage, and the presence of cytogenetic high-risk abnormalities. Logistic regression was performed to evaluate for differences in response between groups. P-values less than 0.05 were considered statistically significant.
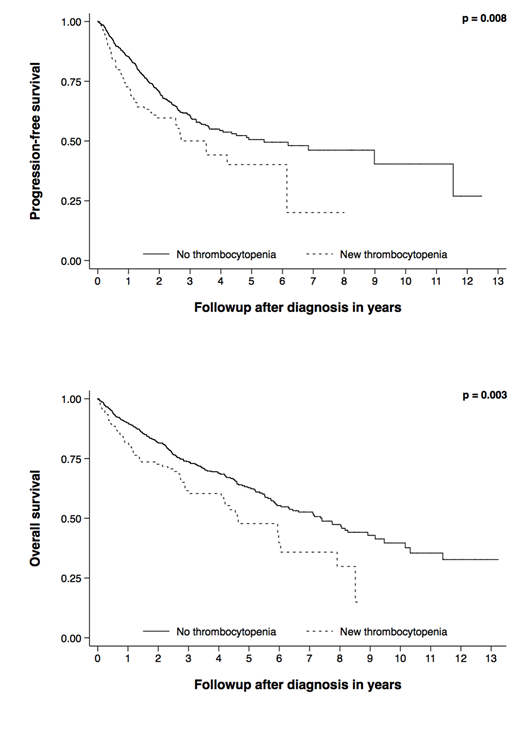
Results: Of the 690 patients treated with novel agents, 495 patients (72%) received a PI as initial therapy. Median follow up was 3 years (0.1-13.2), median overall survival (OS) was 6.6 years (95% CI 5.9-8.0), and median progression free survival (PFS) was 4.84 years (95% CI 3.53-8.98). 123 patients (18%) developed thrombocytopenia within two cycles of first line therapy; two patients developed platelet counts less than 20,000/µL and none developed counts less than 10,000/µL. Differences in survival estimates for OS and PFS for patients who did not develop thrombocytopenia and those who became thrombocytopenic are illustrated in Figure 1. Median OS in patients who did not develop thrombocytopenia was 7.4 years (95% CI 5.9-8.9), compared to 4.6 years (95% CI 4.1-6.0) in those who became thrombocytopenic (p = 0.003), while median PFS was 5.4 years (95% CI 3.63-NR) and 3.53 years (95% CI 0.49-NR), respectively (p = 0.008). Similar results for both OS and PFS were observed when using a platelet nadir of less than 100,000/µL. These relationships remained statistically significant when adjusting for the effect of known prognostic factors including age, sex, ISS stage, and high-risk cytogenetics, for both OS and PFS (HR = 1.70, 95% CI 1.12-1.59, p = 0.013, n = 387 and HR = 1.56, 95% CI 1.03-2.37, p = 0.036, n = 387, respectively). Multivariable-adjusted estimates were similar for the subset of patients receiving PI containing regimens. Development of thrombocytopenia within two cycles was not associated with very good partial response or better (VGPR+) (p = 0.588) or with complete response (CR) (p = 0.968). This was also true for the subset of patients receiving PIs (p = 0.827 and p = 0.772, respectively).
Conclusions: Our results suggest that the development of new, treatment related thrombocytopenia within two cycles of first-line therapy with novel agents is independently associated with inferior survival in newly diagnosed MM. This was also true in the subset of patients receiving PI containing regimens. In contrast to prior studies, there was no association between early treatment-related thrombocytopenia and response to treatment for either novel agents in general or PI based therapies.
Figure 1: Kaplan-Meier progression free and overall survival estimates stratified by platelet count ≥ 150,000/µL (no thrombocytopenia) and <150,000/µL (new thrombocytopenia) within two cycles of initial therapy with novel agents.
Lacy:Celgene: Research Funding. Gertz:Prothena: Honoraria; Teva: Consultancy; Ionis: Honoraria; Alnylam: Honoraria; Research to Practice: Consultancy; Apellis: Consultancy; Physicians Education Resource: Consultancy; janssen: Consultancy; spectrum: Consultancy, Honoraria; Amgen: Consultancy; Abbvie: Consultancy; Medscape: Consultancy; annexon: Consultancy; celgene: Consultancy. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Kumar:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal